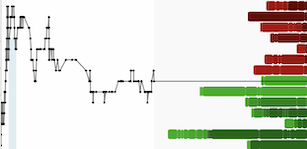
Highly differentiated 1L preliminary systemic activity of 67% ORR and 100% intracranial ORR (by BICR-RANO), including in patients with active brain metastases
45% ORR in 2L patients exceeds competitor benchmarks
Competitive safety profile, with no significant off-target toxicity and manageable on-target toxicity, resulting in low discontinuation rate
Enrollment and follow-up continue in 1L patients at selected dose of 80 mg once daily,
with next update expected mid-2026 ahead of initiation of potential Phase 3 trial
Company to host a conference call and webcast on Saturday, December 6, 2025, at 8:00 pm ET
SOUTH SAN FRANCISCO, Calif. and SAN DIEGO, Dec. 05, 2025 (GLOBE NEWSWIRE) -- ORIC Pharmaceuticals, Inc. (Nasdaq: ORIC), a clinical stage oncology company focused on developing treatments that address mechanisms of therapeutic resistance, announced data from a Phase 1b trial of enozertinib (ORIC-114) at the ESMO Asia Congress 2025. Data in treatment-naïve and in previously treated NSCLC patients with EGFR exon 20 mutations were presented at an oral session, and the presentation can be found in the publication section of ORIC’s website here.
“Enozertinib was purposefully designed to be highly brain-penetrant and exquisitely selective in order to address the limitations of available therapies and potentially drive differentiated durability. These data provide clinical support for this design approach, demonstrating strong systemic and CNS activity in NSCLC patients with EGFR exon 20 mutations,” said Pratik S. Multani, M.D., chief medical officer. “The profile we have seen with enozertinib compares favorably to other approved and investigational therapies and continues to support enozertinib’s best-in-class potential.”
Enozertinib Phase 1b Trial Design
Enozertinib is being evaluated in a Phase 1b trial in patients with locally advanced or metastatic NSCLC with EGFR exon 20 mutations. Notably, enrollment allows patients with active untreated brain metastases. Prior therapy in 2L patients consisted of platinum-based chemotherapy. The primary endpoint of the trial is to determine the recommended Phase 2 dose (RP2D), and secondary endpoints include investigator-assessed objective response rate (ORR), CNS response rate by blinded independent central review (BICR) using response assessment in neuro-oncology (RANO) criteria in treatment-naïve patients, disease control rate (DCR), and safety.
2L NSCLC Patients with EGFR Exon 20 Mutations
As of the August 29, 2025 cutoff date, 45 2L patients were dosed — 24 patients received 80 mg QD oral enozertinib and 21 patients received 120 mg QD. Brain metastases were present in 38% of patients at baseline, including those with active brain metastases.
Preliminary Safety Analysis
Enozertinib was well tolerated with mostly Grade 1 or 2 treatment-related adverse events (TRAEs) and no significant off-target toxicities. Most frequent TRAEs included diarrhea, paronychia, and stomatitis. Only 3 patients discontinued treatment due to TRAEs and higher rates of dose reductions occurred in the 120 mg cohort compared to the 80 mg cohort.
Preliminary Activity Analysis
In the 20 efficacy evaluable patients in the 80 mg cohort, enozertinib demonstrated strong systemic and CNS antitumor activity.
- 45% confirmed ORR and 100% DCR, with comparable rates in patients with brain metastases at baseline
- As of the data cutoff (at a median follow-up of over 30 weeks), 67% of responders remained on treatment
1L NSCLC Patients with EGFR Exon 20 Mutations
As of the August 29, 2025 cutoff date, 33 1L patients were dosed — an initial cohort of 15 patients received 120 mg QD oral enozertinib and a subsequent cohort of 18 patients received 80 mg QD. Brain metastases were present in 39% of patients at baseline, including those with active brain metastases.
Preliminary Safety Analysis
Enozertinib was well tolerated with mostly Grade 1 or 2 TRAEs and no significant off-target toxicities. Most frequent TRAEs included diarrhea, paronychia, and stomatitis. Only 2 patients discontinued treatment due to TRAEs.
The initial cohort of efficacy-evaluable patients was treated at 120 mg QD and 80% of these patients underwent early dose reductions due to TRAEs, such that most of these patients received an effective dose of 80 mg QD. The subsequent cohort of patients was treated at 80 mg QD, for which follow-up is still in progress.
Preliminary Activity Analysis
In the 15 efficacy-evaluable patients in the 120 mg cohort, the majority of which received an effective dose of 80 mg QD, enozertinib demonstrated strong systemic and CNS antitumor activity.
- 67% best ORR (60% confirmed ORR) and 93% DCR
- 100% confirmed intracranial ORR (by BICR-RANO) in patients with measurable CNS disease
- In 4 patients with non-measurable CNS disease, 2 achieved confirmed intracranial complete responses, both with active untreated brain metastases
- As of the data cutoff (at a median follow-up of 33 weeks), 80% of responders remained on treatment
Next Steps
Based on these data, 80 mg QD oral enozertinib has been selected as the dose for potential Phase 3 development. Enrollment and follow-up continues in 1L EGFR exon 20 patients with the next update expected in mid-2026, ahead of initiation of a potential Phase 3 trial.
Conference Call and Webcast Details
In conjunction with the ESMO Asia Congress, ORIC will host a conference call and webcast on Saturday, December 6, 2025, at 8:00 pm ET. To join the conference call via phone and participate in the live Q&A session, please pre-register online here to receive a telephone number and unique passcode required to enter the call. A live webcast and audio archive of the conference call will be available through the investor section of ORIC’s website at www.oricpharma.com. The webcast will be available for replay for 90 days following the presentation.
About ORIC Pharmaceuticals, Inc.
ORIC Pharmaceuticals is a clinical stage biopharmaceutical company dedicated to improving patients’ lives by Overcoming Resistance In Cancer. ORIC’s clinical stage product candidates include (1) ORIC-944, an allosteric inhibitor of the polycomb repressive complex 2 (PRC2) via the EED subunit, being developed for prostate cancer, and (2) enozertinib (ORIC-114), a brain-penetrant inhibitor that selectively targets EGFR exon 20, EGFR atypical, and HER2 exon 20 mutations, being developed across multiple genetically defined cancers. ORIC has offices in South San Francisco and San Diego, California. For more information, please go to www.oricpharma.com, and follow us on X or LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the continued clinical development of enozertinib; statements regarding the potential best-in-class activity or properties of enozertinib, including antitumor activity that exceeds competitor benchmarks; clinical outcomes from studies of enozertinib, which may materially change as patient enrollment continues or more patient data become available; the development plans and timelines for enozertinib; the potential advantages of enozertinib; plans underlying ORIC’s clinical trials and development; next steps and anticipated program milestones, including timing of program and data updates; and statements by the company’s chief medical officer. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon ORIC’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical stage company; ORIC’s ability to develop, initiate or complete preclinical studies and clinical trials for, obtain approvals for and commercialize any of its product candidates; changes in ORIC’s plans to develop and commercialize its product candidates; the potential for clinical trials of enozertinib or any other product candidates to differ from preclinical, initial, interim, preliminary or expected results; negative impacts of health emergencies, economic instability or international conflicts on ORIC’s operations, including clinical trials; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of ORIC’s license and collaboration agreements or its clinical trial collaboration and supply agreements; the potential market for ORIC’s product candidates, and the progress and success of competing therapeutics currently available or in development; ORIC’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; regulatory developments in the United States and foreign countries; ORIC’s reliance on third parties, including contract manufacturers and contract research organizations; ORIC’s ability to obtain and maintain intellectual property protection for its product candidates; the loss of key scientific or management personnel; competition in the industry in which ORIC operates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in ORIC’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the SEC) on November 13, 2025, and ORIC’s future reports to be filed with the SEC. These forward-looking statements are made as of the date of this press release, and ORIC assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Contact:
Dominic Piscitelli, Chief Financial Officer
dominic.piscitelli@oricpharma.com
info@oricpharma.com