Ferring Pharmaceuticals and Theralase® Technologies Inc. (TSX-Venture: TLT) (OTCQB: TLTFF) have entered into a clinical development collaboration to study a potential combination treatment for patients with high-risk, BCG-unresponsive non-muscle invasive bladder cancer (NMIBC). The study will evaluate Ferring’s FDA-approved gene therapy ADSTILADRIN® alongside Theralase®’s investigational light-activated drug Ruvidar® (TLD-1433) in patients with carcinoma in situ, with or without papillary tumors. The goal is to assess whether combining these two therapies can expand treatment options for a patient population with limited alternatives.
The agreement, signed on January 9, 2026, builds upon Theralase®’s ongoing clinical program and introduces a new patient cohort focused on the combination approach. Theralase® will continue as the study sponsor, while both companies will jointly oversee development through a shared committee. Initial enrollment and treatment will take place in the United States, with the possibility of expanding into Canada or other regions pending further agreement.
This collaboration highlights a shared commitment by both companies to advancing innovative bladder cancer therapies through complementary mechanisms of action. ADSTILADRIN® works by delivering a gene that enables bladder cells to produce interferon, triggering localized anticancer and immune responses. Ruvidar®, meanwhile, is designed to selectively enter cancer cells and once activated by light, destroy them while stimulating both innate and adaptive immune activity. Together, the therapies aim to enhance therapeutic effectiveness for patients who have not responded to standard BCG treatment.
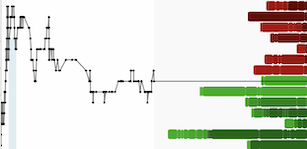
Shares of TLT are currently up 46.94%, while U.S. listed shares (TLTFF) are surging 46.77% in mid-morning trading.
Copyright © 2025 AllPennyStocks.com. All rights reserved. Republication or redistribution of AllPennyStocks.com's content is expressly prohibited without the prior written consent of AllPennyStocks.com. AllPennyStocks.com shall not be liable for any errors or delays in the content, or for any actions taken in reliance thereon.
View more of this article on AllPennyStocks.com.
About AllPennyStocks.com Media, Inc.:
Founded in 1999, AllPennyStocks.com Media, Inc. is North America's largest and longest running website dedicated exclusively to micro-cap and small-cap insights.
Catering to both Canadian and U.S. markets, AllPennyStocks.com provides a wealth of resources and expert content designed for everyone, from beginner investors to seasoned traders.
AllPennyStocks.com's content is prominently featured across numerous top-tier financial platforms, reaching a broad audience of investors and industry professionals.
Contact:
AllPennyStocks.com Media, Inc.
Email: ads@allpennystocks.com
Phone: (800) 558-4560 Ext: 101
]]>