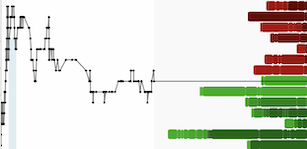
Inspira Technologies announced positive results from the clinical evaluation of its AI-powered HYLA blood sensor. Inspira believes that these breakthrough results strengthen the Company’s progress in developing its innovative real-time blood monitoring capabilities, as a means of enabling early diagnosis and personalized care without the need for intermittent blood draws.
The Company is proud to report that the HYLA successfully achieved over 96% accuracy, as compared to traditional blood test analyzers.
The Company anticipates that these new results will support the advancement towards its expected Food and Drug Administration (FDA) submission of the first HYLA configuration in the second half of 2025. Once FDA clearance is obtained, the Company plans to deploy this core technology of the INSPIRA ART in U.S. hospitals for data collection and post-market validation.