In a biotech sector crowded with speculative plays and distant promises, Quantum BioPharma (NASDAQ: QNTM) stands out as a focused, de-risked, and strategically diversified contender. With a first-in-class drug candidate for multiple sclerosis and a near-term revenue engine in consumer wellness, Quantum is rapidly assembling a unique platform that could reshape both chronic neurodegeneration and acute alcohol recovery.
$QNTM is delivering on the rare convergence of scientific innovation, market timing, and operational precision. For investors seeking exposure to transformative healthcare, Quantum isn’t just another early-stage story, it’s a breakout opportunity hiding in plain sight.
A Therapeutic Breakthrough for MS: Lucid-MS Takes the Lead
At the core of Quantum’s pipeline is Lucid-MS (Lucid-21-302), a drug candidate that could redefine the treatment of multiple sclerosis. While most MS therapies focus on suppressing the immune system to slow disease progression, Lucid-MS takes aim at the underlying neurological damage itself. Its goal is ambitious but essential: to repair myelin, the protective sheath around nerves, and potentially reverse functional decline in MS patients.
Lucid-MS works by inhibiting PAD enzymes—molecular culprits that degrade myelin integrity in the central nervous system. In preclinical models, the compound didn’t just slow disease—it restored motor function, indicating real regenerative potential. In a Phase 1 clinical trial completed in Australia, the compound showed no serious adverse events, clearing a critical safety hurdle and positioning Quantum to advance to Phase 2 with confidence.
Now, as Quantum prepares to initiate Phase 2 trials, it is doing so from a position of operational and financial strength. In its Q2 2025 financial results, reported on August 7, the company confirmed that it had successfully completed all oral toxicity studies of Lucid-MS with no adverse effects, while also launching a potentially groundbreaking imaging study in collaboration with Massachusetts General Hospital. The first MS patient has already been scanned using a PET imaging technique that could offer a real-time biomarker for remyelination—a potential first in neurology.
Meanwhile, Quantum submitted Lucid-MS to the UK’s ILAP fast-track pathway and is preparing an IND filing with the U.S. FDA, expected in Q4 2025. With both safety and technical validation in place, Lucid-MS is on track to become a first-in-class oral treatment offering genuine neuroprotection for MS patients.
Why Lucid-MS Could Be a Billion-Dollar Asset
The market potential here is undeniable. There are nearly 2.8 million MS patients globally, and the existing therapies—despite their cost—do not stop or reverse disability progression. The MS drug market generated $28.2 billion in 2022, and is forecast to surpass $41 billion by 2033. Even a niche approval in progressive MS could deliver outsized returns, especially given the premium pricing associated with novel CNS therapies.
Lucid-MS may also be non-immunosuppressive, meaning it could be used alongside current therapies, broadening its reach. And because PAD enzyme overactivity is implicated in other demyelinating diseases, Quantum may be holding a pipeline-in-a-product with even wider neurological applications.
The Consumer Catalyst: Unbuzzd™ Launches, Spins Out, and Scales
While Lucid-MS builds long-term value, Quantum is already generating short-term commercial traction through Unbuzzd™, a clinically validated beverage that helps accelerate alcohol metabolism and reduce hangover symptoms. Launched in late 2024 and now available on Amazon and direct-to-consumer, Unbuzzd is positioned at the intersection of functional wellness and recreational recovery.
Through its wholly owned licensee, Unbuzzd Wellness Inc., Quantum retains a ~20% equity stake and receives royalties on sales (7% up to $250 million USD). As disclosed in Q2 filings, the brand has already launched a Regulation D 506(c) raise for up to $5 million USD to fund retail expansion and a planned IPO, now being prepared with MNP auditors.
Kevin Harrington’s unbuzzd Revolution: How This Will Change Society | Part 3
This structure provides near-term, non-dilutive upside for Quantum shareholders while opening the door for future monetization. Notably, Unbuzzd’s revenue is growing organically quarter over quarter, and the Canadian application for Health Canada approval is pending—potentially opening a second national market for expansion.
A Clinical-Grade Alcohol Antidote: The REKVRY Program
Building on the Unbuzzd platform, Quantum is advancing REKVRY, a clinical-grade therapeutic for acute alcohol intoxication. Unlike current emergency care protocols—which rely solely on time and hydration—REKVRY is designed to actively reduce blood alcohol concentration and mitigate symptoms of poisoning.
$QNTM’s METAL-2 trial, expected to report results in the second half of 2025, aims to validate the efficacy of the formulation in a clinical setting. If successful, REKVRY could revolutionize care in emergency rooms, ambulances, military settings, and remote medicine. It would also validate Unbuzzd’s mechanism of action and unlock further regulatory pathways.
Strategic Collaborations, Strong Balance Sheet, and Share Price Momentum
Unlike many small-cap biotechs, $QNTM is executing from a position of financial strength. In Q2 2025, the company:
Increased total current assets to $10.3 million USD, up from $9.9 million USD in Q1
Converted all outstanding debentures into equity, eliminating debt-related liabilities
Maintained a 'no going concern' status, with sufficient cash to operate through March 2027
Recorded over $500,000 USD in gains from its $5.5 million USD crypto portfolio
Received a $2.35 million USD settlement from a prior legal matter
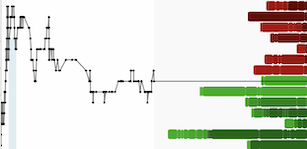
Reported a nearly threefold increase in share price, rising from $7.71 USD to $20.25 USD between March and June 2025
Importantly, the company now carries no warrant liability, and recent increases in operating expenses were largely driven by non-cash share-based payments and legal administration, not increased burn.
Twin Engines, One Unified Theme: Brain Health
Though seemingly distinct, Quantum’s two programs—neurodegeneration and alcohol detox—share a unifying vision: restoring clarity, resilience, and function in the human brain. This thematic consistency not only positions Quantum at the forefront of “brain health,” but also creates diversified market exposure across both chronic CNS conditions and high-volume acute care.
Together, the MS and alcohol markets represent tens of billions in addressable opportunity. Yet Quantum's current valuation reflects none of the upside from Lucid-MS's Phase 2 potential, nor from Unbuzzd's consumer breakout or REKVRY’s future clinical deployment. That disconnect is the essence of the bull case.
Key Near-Term Catalysts Include:
Mid to Late 2025: Potential entry into the UK’s ILAP program for Lucid-MS, accelerating regulatory timelines
Q4 2025: Submission of IND application for Lucid-MS to begin Phase 2 efficacy trials in the U.S.
H2 2025: Readout from METAL-2 trial, evaluating alcohol detox formulation in human volunteers
2025 (TBD): IPO or strategic monetization event for Unbuzzd Wellness Inc.
Ongoing: Retail expansion updates for Unbuzzd™ and Health Canada decision
2025 Observational Data: Progressive MS study in Australia to support trial design
As always with early-stage biotech, ongoing regulatory developments and trial outcomes will be critical determinants of long-term value.
Final Word: The Market Is Still Pricing
Quantum BioPharma is assembling a rare combination: a de-risked clinical asset with blockbuster potential, a launched consumer product already in revenue mode, and a high-value clinical pipeline for alcohol-related emergencies. Backed by top-tier partners, a strengthened balance sheet, and a seasoned regulatory team, Quantum is building a multi-vertical, high-leverage platform—all underpinned by a clear focus on brain health.
CEO Zeeshan Saeed put it plainly following the company’s record-breaking second quarter:
“We finished Q2 2025 in an even stronger position than Q1, with a fortified balance sheet, strategic execution across our MS and Unbuzzd programs, and three potential near-term monetization events. Lucid-MS continues to show promise as a first-in-class, non-immunomodulatory compound, while Unbuzzd has launched a $5 million raise ahead of a potential go-public transaction—without diluting Quantum shareholders. With strategic cryptocurrency diversification, meaningful cash runway, and expanding market presence, we believe the significant rise in our share price reflects confidence in the work we’ve done and what’s still to come.”
For investors, the opportunity here is two-fold: ride the revaluation wave as milestones unfold in 2025, and participate early in what could become a dominant player in remyelination therapy and alcohol recovery science.
This is not a long shot. It’s a high-conviction, high-reward setup. And it’s unfolding now.